
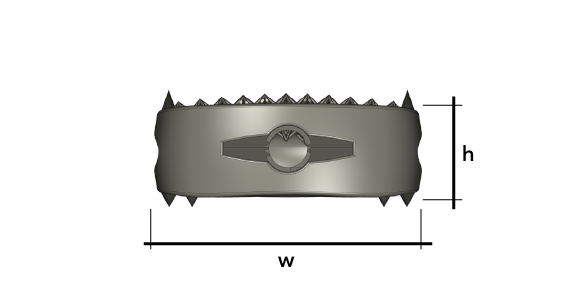
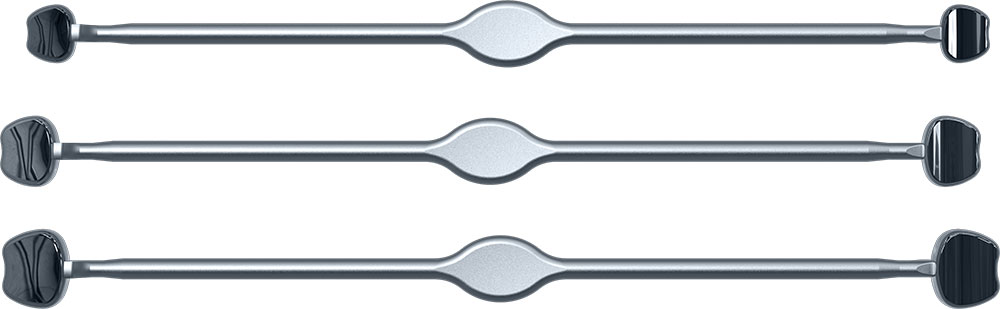
Dynam’X Aries and related instruments set is dedicated for Anterior Cervical Discectomy and Fusion (ACDF) procedure. Aries Cage incorporates multispikes titanium net which allows free flow of bone cells. Aries is designed to create “prepress” of the elastic net on vertebral endplates. Aries System possibility to fill cage by autograft.
Dynam’X Aries ACDF Cage has high coherence to cervical spine anatomy in axial & sagittal plane and offers superior diagnostic compatibility including MRi surveys. Due to IsoFrame® application, metal volume has been significantly reduced while maintaining durability and mechanical properties. Incorporated Syntropiq DCS ® Technology initiates and accelerates bone remodeling process, that results in short fusion time and massive creation of new bone scaffolds 1)
Dynam’X Aries has built-in primary stabilization system and allows filling with bone substitute either by paste or granules formulations.
Dynam’X System is indicated for intervertebral body fusion procedures in skeletally mature patients which have had six months of non-operative therapy. The intended use is to recreate the intervertebral disc hight and to give structural support for loading forces during the healing period and by design purpose, it supports fusion process. See IFU for indications, contraindications and other details.
1) Subject of clinical investigation under “Dynam’X Records” PMCF European Study
Dynam’X Aries ACDF Cage has high coherence to cervical spine anatomy in axial & sagittal plane and offers superior diagnostic compatibility including MRi surveys. Due to IsoFrame® application, metal volume has been significantly reduced while maintaining durability and mechanical properties. Incorporated Syntropiq DCS ® Technology initiates and accelerates bone remodeling process, that results in short fusion time and massive creation of new bone scaffolds 1)
Dynam’X Aries has built-in primary stabilization system and allows filling with bone substitute either by paste or granules formulations.
Dynam’X System is indicated for intervertebral body fusion procedures in skeletally mature patients which have had six months of non-operative therapy. The intended use is to recreate the intervertebral disc hight and to give structural support for loading forces during the healing period and by design purpose, it supports fusion process. See IFU for indications, contraindications and other details.
1) Subject of clinical investigation under “Dynam’X Records” PMCF European Study
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1012140204 | 1012140604 |
| 5 mm | 1012140205 | 1012140605 |
| 6 mm | 1012140206 | 1012140605 |
| 7 mm | 1012140207 | 1012140605 |
| 8 mm | 1012140208 | 1012140605 |
| 9 mm | 1012140209 | 1012140605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1014170204 | 1014170604 |
| 5 mm | 1014170205 | 1014170605 |
| 6 mm | 1014170206 | 1014170605 |
| 7 mm | 1014170207 | 1014170605 |
| 8 mm | 1014170208 | 1014170605 |
| 9 mm | 1014170209 | 1014170605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1016190204 | 1016190604 |
| 5 mm | 1016190205 | 1016190605 |
| 6 mm | 1016190206 | 1016190605 |
| 7 mm | 1016190207 | 1016190605 |
| 8 mm | 1016190208 | 1016190605 |
| 9 mm | 1016190209 | 1016190605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1112140204 | 1112140604 |
| 5 mm | 1112140205 | 1112140605 |
| 6 mm | 1112140206 | 1112140605 |
| 7 mm | 1112140207 | 1112140605 |
| 8 mm | 1112140208 | 1112140605 |
| 9 mm | 1112140209 | 1112140605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1114170204 | 1114170604 |
| 5 mm | 1114170205 | 1114170605 |
| 6 mm | 1114170206 | 1114170605 |
| 7 mm | 1114170207 | 1114170605 |
| 8 mm | 1114170208 | 1114170605 |
| 9 mm | 1114170209 | 1114170605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1116190204 | 1116190604 |
| 5 mm | 1116190205 | 1116190605 |
| 6 mm | 1116190206 | 1116190605 |
| 7 mm | 1116190207 | 1116190605 |
| 8 mm | 1116190208 | 1116190605 |
| 9 mm | 1116190209 | 1116190605 |


| h | 2° | 6° |
|---|---|---|
| 4 mm | 1212140204 | 1212140604 |
| 5 mm | 1212140205 | 1212140605 |
| 6 mm | 1212140206 | 1212140605 |
| 7 mm | 1212140207 | 1212140605 |
| 8 mm | 1212140208 | 1212140605 |
| 9 mm | 1212140209 | 1212140605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1214170204 | 1214170604 |
| 5 mm | 1214170205 | 1214170605 |
| 6 mm | 1214170206 | 1214170605 |
| 7 mm | 1214170207 | 1214170605 |
| 8 mm | 1214170208 | 1214170605 |
| 9 mm | 1214170209 | 1214170605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1216190204 | 1216190604 |
| 5 mm | 1216190205 | 1216190605 |
| 6 mm | 1216190206 | 1216190605 |
| 7 mm | 1216190207 | 1216190605 |
| 8 mm | 1216190208 | 1216190605 |
| 9 mm | 1216190209 | 1216190605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1312140204 | 1312140604 |
| 5 mm | 1312140205 | 1312140605 |
| 6 mm | 1312140206 | 1312140605 |
| 7 mm | 1312140207 | 1312140605 |
| 8 mm | 1312140208 | 1312140605 |
| 9 mm | 1312140209 | 1312140605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1314170204 | 1314170604 |
| 5 mm | 1314170205 | 1314170605 |
| 6 mm | 1314170206 | 1314170605 |
| 7 mm | 1314170207 | 1314170605 |
| 8 mm | 1314170208 | 1314170605 |
| 9 mm | 1314170209 | 1314170605 |
| h | 2° | 6° |
|---|---|---|
| 4 mm | 1316190204 | 1316190604 |
| 5 mm | 1316190205 | 1316190605 |
| 6 mm | 1316190206 | 1316190605 |
| 7 mm | 1316190207 | 1316190605 |
| 8 mm | 1316190208 | 1316190605 |
| 9 mm | 1316190209 | 1316190605 |
| size | type | 2° | 6° |
|---|---|---|---|
| SMALL | 4-5 | 102016B | 107016B |
| 6-7 | 102017B | 107017B | |
| 8-9 | 102018B | 107018B | |
| MEDIUM | 4-5 | 102019B | 107019B |
| 6-7 | 102020B | 107020B | |
| 8-9 | 102021B | 107021B | |
| LARGE | 4-5 | 102022B | 107022B |
| 6-7 | 102023B | 107023B | |
| 8-9 | 102024B | 107024B |












The knowledge we obtained from our extensive research allowed us to create exceptional spine implants. Find out more about our unique technologies used in them.
It is one of the best materials for bone fusion in the medical industry. The low density allows MRi and CT diagnostic compatibility.
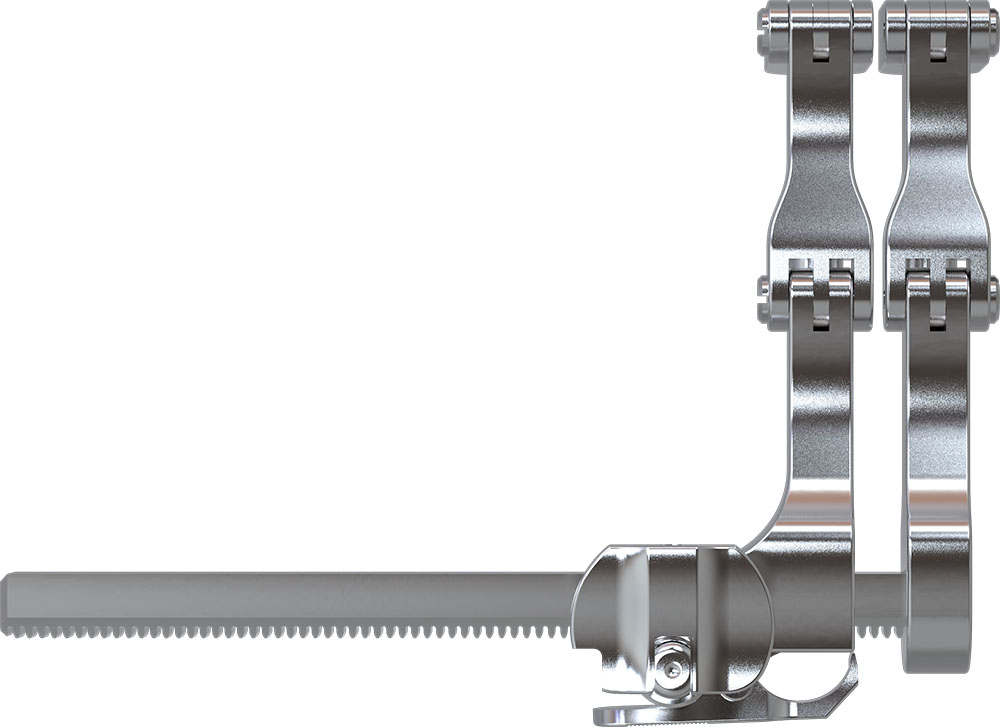
Read MoreDynam’X System design based on a circumferential frame which bears the physiological loads and a specially engineered multi-spiked dynamic contact surface that increases the contact area and actively promotes bone ingrowth process.
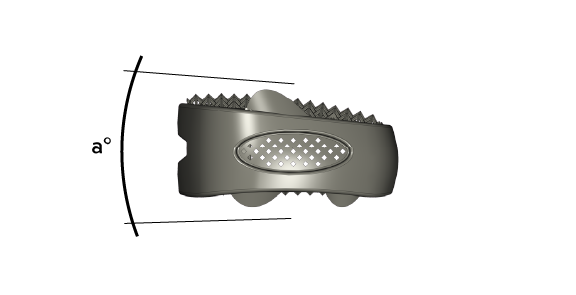
Read Moreto avoid sagittal imbalance in spine our broad product portfolio offers different angulations of the cages including hyperlordotic cages.
Read Morethe cage’s design allows acceleration of fusion by controlled subsiding zone on the implant-endplate area. This phenomenon stimulates dynamization of the bone remodeling process in accordance with Wolff’s Law.
Read MoreIf you have any questions about our products or the technologies we use feel free to contact us via the contact form.
If you are interested in becoming our business partner see more information here.

Unique Three Dimensional Design of Syntropiq Dynamic Contact Surface distributes Isotropically Physiological Loads and Contributes Proactively in Bone Ingrow Process
Syntropiq DCS employs Controlled Subsiding Process and by following the Cellular Mechanism of Bone Remodeling initiates Bone Cells Reversal Process
By engaging Wolf’s Law accelerates Growing Process of New Bone Structures
Straight Insertion Path
Anti Rotation
Harmless Rails on Endplate
Free Flow of Blood for Supplying of New Bone Structures
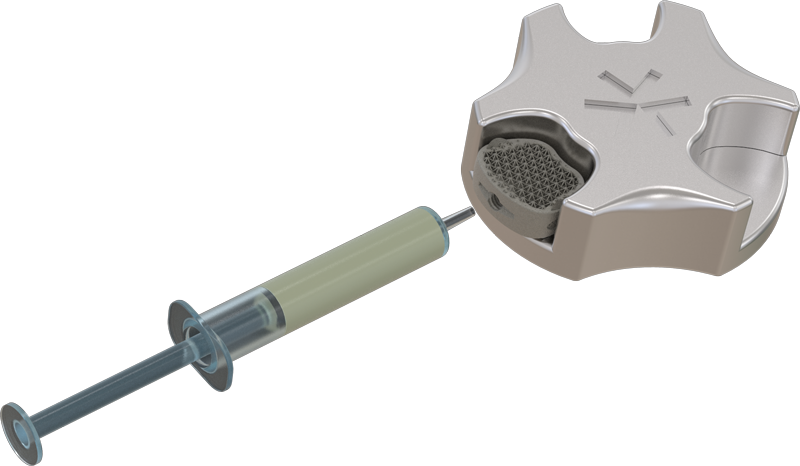
Aries – Model Regular
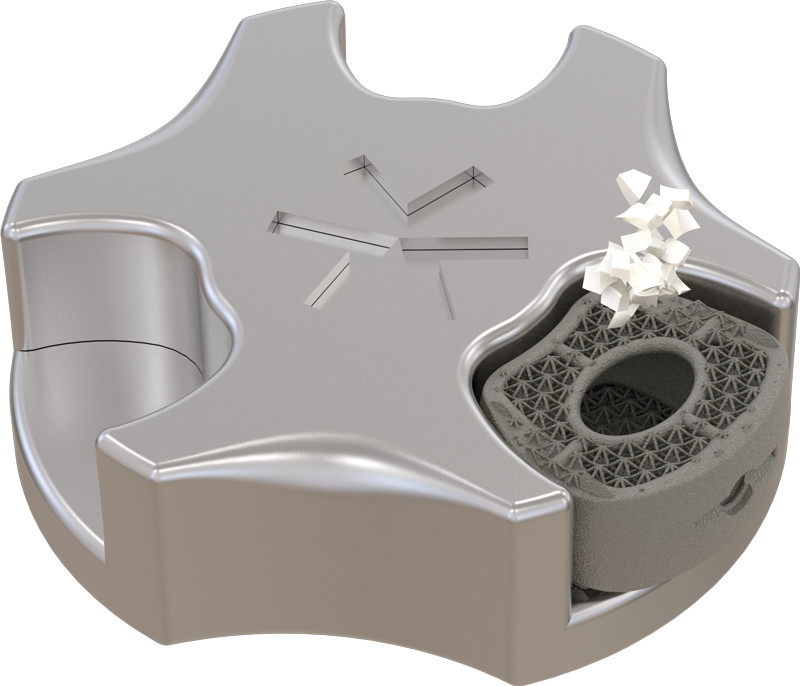
Aries – Model Hollowed
Grafting options for bone substitutes or bone chips available